
EMA accepts regulatory submission for avalglucosidase alfa.
Avalglucosidase alfa reaches its first important regulatory milestone

Avalglucosidase alfa reaches its first important regulatory milestone
AGSD-UK working with NICE towards approval for new treatment for Pompe disease.

A survey looking at the Covid-19 impact on wellbeing in families of children with rare neurodevelopmental and genetic disorders study

Audentes is now enrolling patients with late-onset Pompe disease in the Phase 1/2 clinical trial.
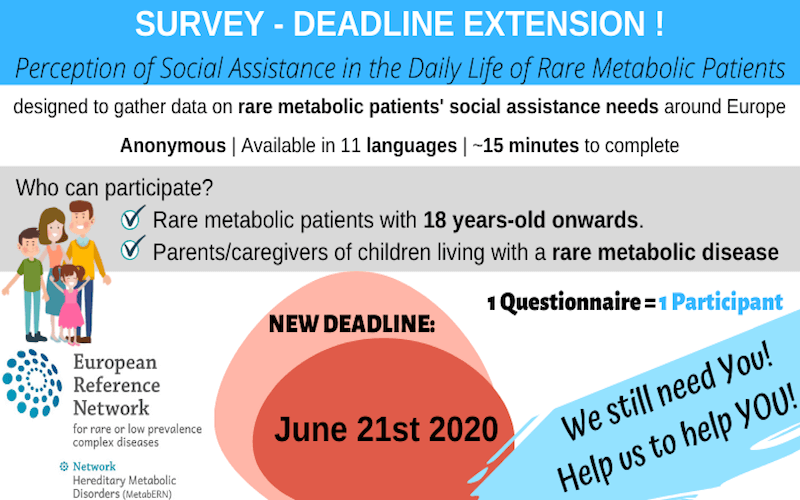
MetabERN has extended its survey deadline to June 21st 2020

Dr. Weinstein, long-time champion of improved diagnosis and treatment for hepatic GSDs due to move to Passage Bio as VP.